Examining the regulatory nuances of Canada and the U.S.

Although several differences exist between the two countries, Canada and the United States also share many similarities. This is especially true when it comes to the regulatory environments governing the health care facilities in each country.
Health care facilities in Canada are governed by national and provincial codes and regulations. In addition, Canadian facilities are regulated by a set of health care facility standards developed by the Canadian Standards Association (CSA) Group.
CSA Group is a global organization dedicated to safety, social good and sustainability. It is a leader in standards development, and in testing, inspection and certification not just in Canada but in the U.S., Europe and Asia, as well. Its mandate is to “hold the future to a higher standard.” The mission of CSA’s standards development branch is to “enhance the lives of Canadians through the advancement of standards in the public and private sectors.” These health care standards are an important part of keeping patients and staff safe.
A thorough review
The CSA standards are written by a group of volunteers serving on technical subcommittees (TSC). These volunteers meet to review and amend existing standards, and develop new standards. The life cycle of these standards is completed on a five-year basis, excluding special circumstances. The TSCs are composed of individuals representing health care facilities, manufacturers, consultants, contractors and a project manager from CSA. The starting point for revision of a standard is typically one of the following factors: incidents in existing facilities that have compromised the safety of patients, visitors and staff; improvements to the standard identified by TSC members; official inquiries submitted to CSA; and finally, since many of the subcommittee members are also instructors, questions gathered from participants during courses conducted by CSA.
The work of the committees is divided into small working groups that diligently discuss and develop potential changes. These changes are brought back to the entire TSC with proposals for review and discussion. Any emerging revisions are then prepared into a final draft, which is circulated among the subcommittee members for this step’s final review. The members crosscheck for any overlap with other standards to ensure accuracy, and any disagreements are resolved by consensus.
Once the draft is ready, it is posted for a public comment window of 60 days to allow for input from concerned public stakeholders. At the end of the 60 days the input is collated, and the comments are reviewed by the TSC. Changes are either rejected, accepted as-is or accepted with some revision. Afterward, the TSC reviews the final draft again.
Once the TSC has completed this portion of work, the draft heads to the CSA’s Technical Committee for Health Care Facilities, where members vote on its applicability. In turn, a strategic steering committee (SSC) (in the case of health care that would be the SSC for Health Care and Well-being) must be balloted for applicability and transparency of process. The technical committee and SSC are required to maintain a balance of members, including users, manufacturers, regulatory experts and public interest stakeholders. No one group is permitted to overpower the other groups in the voting process or committee makeup.
Some of the standards applicable to health care include infection control during construction, renovation, maintenance and repair; emergency electrical power supply; special requirements for heating, ventilating and air conditioning systems; and electrical safety and essential electrical systems.
It is important to keep in mind that the standards are a minimum requirement and not all standards are legally required to be followed. However, some of the standards are referenced in federal and provincial building codes or regulations, which makes them a legislated requirement. Some of the more recent standards developed include CSA Z317.12:20, Cleaning and disinfection of health care facilities, and CSA Z8004:22, Long-term care home operations and infection prevention and control. The latter standard was driven by the lessons learned in long-term care homes during the COVID-19 pandemic.
Building commonalities
There are many similarities between CSA standards and U.S. regulations, codes and standards. For example, the CSA Z7396.1:22, Medical gas pipeline systems – Part 1: Pipelines for medical gases, medical vacuum, medical support gases, and anaesthetic gas scavenging systems, is similar to medical gas requirements found in the National Fire Protection Association’s NFPA 99, Health Care Facilities Code.
Another CSA standard with a familiar U.S. equivalent is CSA Z8000:18, Canadian health care facilities, which provides information on constructing and renovating health care facilities. This standard resembles the Facility Guidelines Institute’s Guidelines for Design and Construction of Hospitals and the Guidelines for Design and Construction of Outpatient Facilities. In fact, the FGI Guidelines are included in the reference documents in the latest version of CSA Z8000, Building for health and safety in our health care facilities. The CAN/CSA-Z317.13-17, Infection control during construction, renovation, and maintenance of health care facilities, mandates an infection control risk assessment, similar to U.S. regulations. A final example is that NFPA regulations are used in many Canadian fire and building codes.
Marked by differences
One major difference between Canada and the United States is that accrediting organizations (AOs) in Canada, such as Accreditation Canada, do not always reference the CSA health care facilities standards in many of their required organizational practices. This leads to issues for health care facilities managers in Canada working to ensure that the standards are being followed. During new construction or renovations, the standards are met in most cases. However, since the operation and maintenance portion of the standards is a voluntary objective, many facilities — lacking funds for staff, contractors and materials — may forego the work required to meet all the standards’ operational aspects.
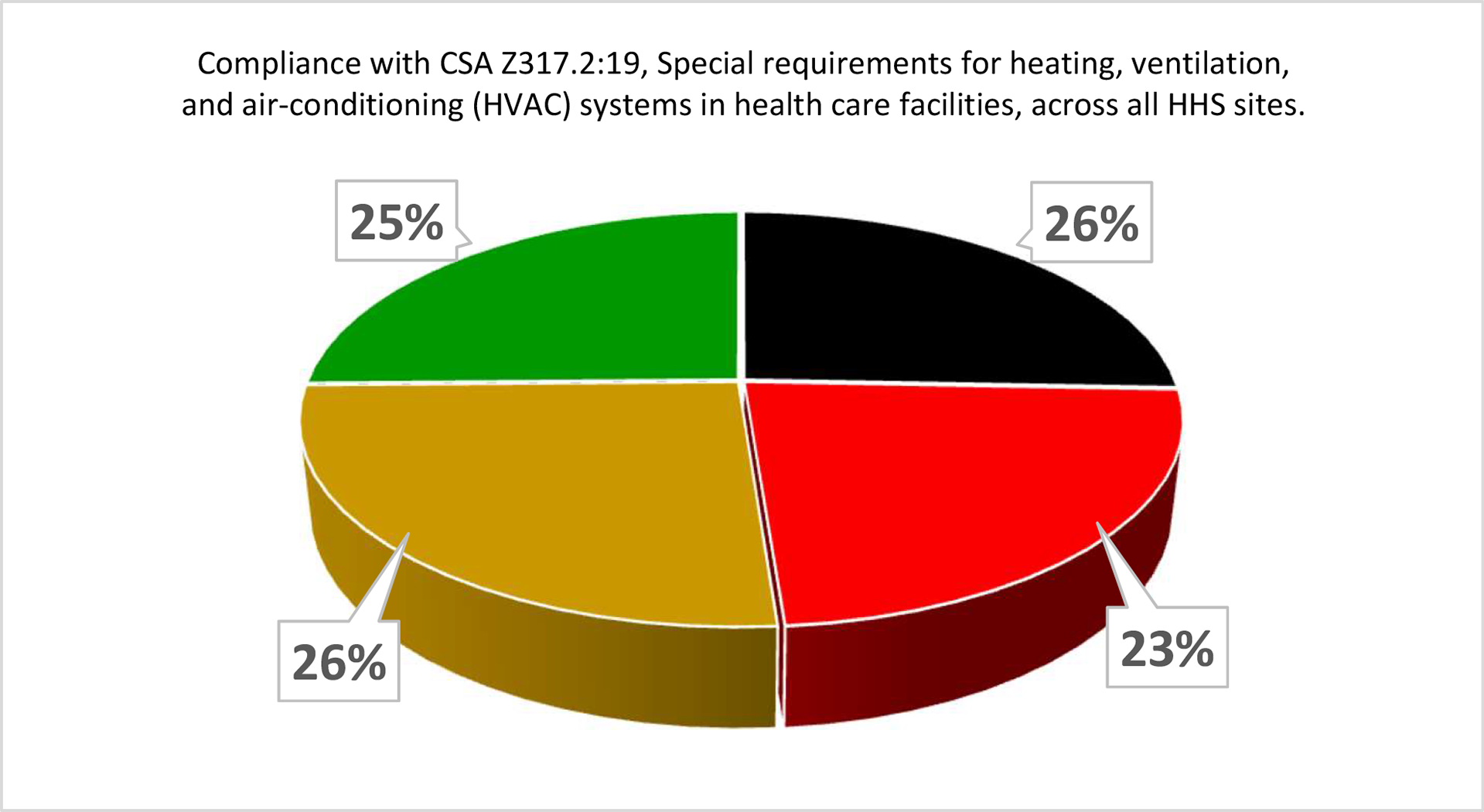
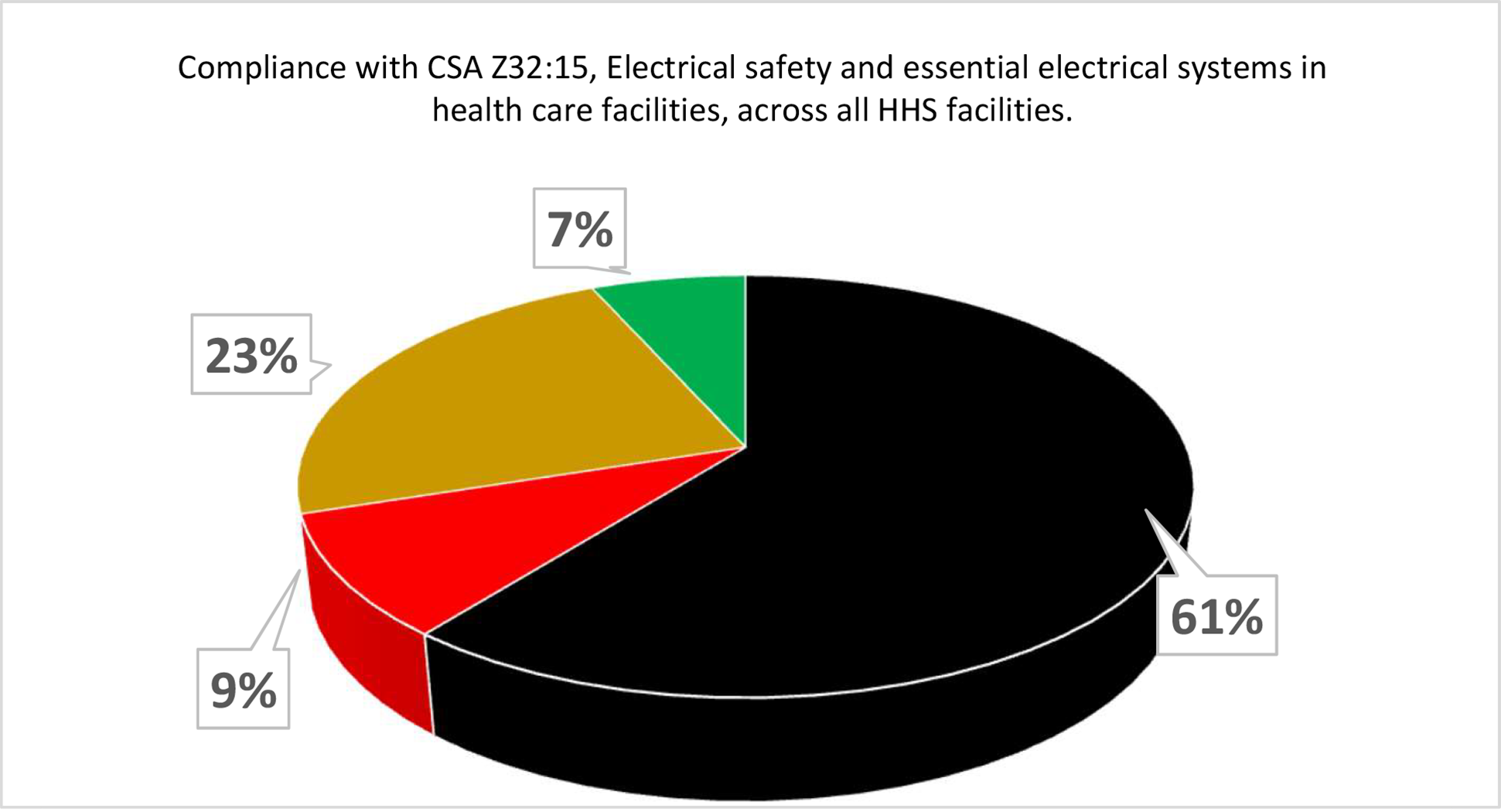
At Hamilton Health Sciences (HHS), a large, five-hospital system in Hamilton, Ontario, a CSA scorecard was developed to provide a comparison of actual compliance versus required compliance for all CSA standards relating to health care operations and management. As far as HHS is aware this was the first time a Canadian Health System has attempted this. Each standard was researched to identify an operations and management requirement, and a table was developed to show where the organization stood at any point in time compared to that requirement. A simple green, yellow, red and black scoring system was used as per the following guidelines for each score:
- Green. 100% compliant and documented
- Yellow. 50-99% compliant with some documentation
- Red. 1-49% compliant with minor documentation
- Black. 0% compliant and no documentation
Each site was scored using a review of documented tests and reports, supervisors’ feedback and building walkthroughs. The results were surprising to many of the facilities management leadership staff.
As can be seen by the two examples (above and below), there is a lot of work to be done to move all requirements to green. The plan is to assess the progress of the scorecard and rescore each category on an annual basis. In addition, as new standards are published, they will need to be reviewed and scored.
While both Canada and the U.S. require health care facilities to meet a unique set of standards, one commonality between the two countries is that it is incumbent upon the facilities management and capital assets professionals to ensure their facilities are meeting all requirements during their AO surveys. Use of a scorecard with a dashboard can provide a quick view of a health system’s compliance status and is an excellent tool to help achieve compliance no matter where the facility resides.
George Pankiw, P.Eng, CCHFM, SASHE, CHFM, is director of capital development at Hamilton Health Sciences in Hamilton, Ontario. He can be reached at pankiwg@hhsc.ca.