Open communications

Imagine a facility where trending information is automatically transferred from a ventilator into an electronic health record (EHR). Doctors can view data directly from the ventilator, identify more spontaneous breathing, and consult with the respiratory therapist regarding a weaning protocol, all without ever having to call the nurse or visit the patient.
This has long been a possibility, but is now becoming a reality. Hospitals are seeing data pulled from medical devices such as physiologic monitors, vital signs monitors, ventilators and infusion pumps and transferred automatically into the EHR. Unfortunately, this is not as easy as inserting a plug into a computer.
This is where medical device connectivity solutions come into play. They allow data to be transferred from the medical device to the EHR, while solving such issues as information mapping and patient association/disassociation.
But how will these connectivity solutions affect a health care facility? What planning and discussions need to take place to ensure that no data are lost? What infrastructure questions should be asked to make sure no problems arise? These questions and more need to be answered before a health care facility decides to move ahead with medical device integration.
Reasons for connectivity
The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act calls for hospitals to adopt meaningful use of their EHRs through a set of objectives that a health care facility must meet to qualify for Centers for Medicare & Medicaid Services incentive payments. Part of transitioning to meaningful use for health care facilities is capturing comprehensive data from individual medical devices for better use by clinicians.
Medical devices rarely can be connected directly to the EHR to allow a free-flowing exchange of information. This is due to the differences in types of information being transferred and the format in which this information is displayed. If the fields within the medical devices and the EHR were all standardized, information transfer would be less complicated.
Given the way information is stored and displayed, a medical device connectivity solution is necessary to help connect medical devices to the EHR. For example, an infusion pump cannot transfer information directly to an EHR but, with the help of a connectivity solution, an infusion pump and an EHR can exchange information.
Currently, connectivity solutions are available from medical device vendors, EHR vendors and third-party vendors. Typically, solutions from medical device vendors and EHR vendors work with only a small number of situations (e.g., their device into the most popular EHRs) and, therefore, only cover some of the vast combinations that can occur. For this reason, most health care facilities find these solutions undesirable.
In contrast, third-party vendors are intended to be medical device- and EHR vendor-neutral. This can be advantageous because a single vendor would manage the interfaces between the medical device data and the EHR system, rather than requiring the facility to manage multiple interfaces for each type of medical device. A common interface potentially decreases cost and complexity in a health care facility.
Working as middleman
Ultimately, medical device connectivity solutions work as the middleman between the medical device and the hospital information system. They typically comprise a point-of-care (POC) component, which associates the patient to the medical device while collecting the data from the medical device and sending it to the server. The server then interfaces with the EHR-hospital information system to facilitate communication between the medical device data and the EHR.
The automation of data transfer has a multitude of benefits for a health care organization, but the main reasons can be summarized as:
Increased efficiency and improved workflow. The most important and visible improvement when integrating medical devices to an EHR is the increased efficiency of staff. Not having to manually enter all patient data into the EHR will save staff time, energy and money better spent on patient care. Imagine a clinician's needing the latest vital sign reading to make a diagnosis on a patient. Instead of running to the chart to check, what if all of the information was automatically uploaded and at his or her fingertips for review? Removing menial tasks not only reduces the potential for risk, but it also creates a potential for more quality time to be spent with patients. Overall, integrating the medical devices and the EHR to create an automatic data transfer can help your facility standardize workflows and maximize provider efficiency.
Improved communication. Without medical device integration, medical information is scattered throughout the organization. Some patient information can be found in the EHR, while other vital information is in the medical device, patient chart or even in the clinician's thoughts. Automating the transfer of data from the medical device to the EHR helps to improve communication and reduce the scatter of information. Because most of the data are available in the EHR, communication problems are less likely to occur. Clinicians and providers are more likely to have all the available information, which can help in making the best diagnostic decisions.
These advantages and benefits illustrate that health care services provided to patients are dramatically enhanced. A reduction in manual entry errors gives doctors more accurate information so they can make better diagnoses. Physicians and nurses also can focus more on patient care, instead of worrying about inputting data. This improves both patient safety and patient satisfaction.
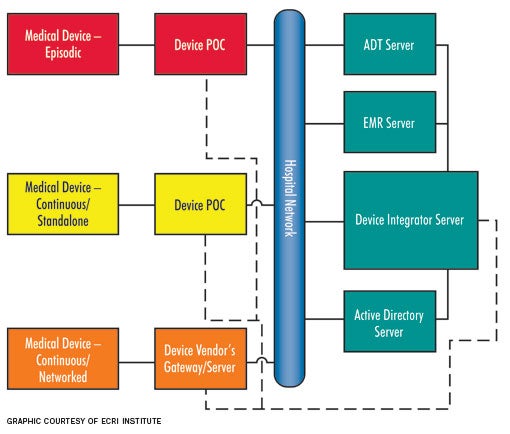
State of connectivity
Connectivity solutions are available from a variety of vendors. However, different vendors achieve integration in different ways. Some vendor solutions may include both hardware and software components, while other vendors may provide only a software solution. While vendor approaches to integration can be different, all solutions are based on characterizing the medical device as either an "episodic" or a "continuous" device. Continuous devices either can be networked or non-networked or stand-alone, although this depends mostly on how the patient data are sent from the medical device to the EHR.
Episodic devices. Episodic devices obtain a single set of measurements from a patient at a given time (i.e., spot checks). The most commonly considered episodic device is a vital-signs monitor. To achieve integration, a vital-signs monitor records multiple physiologic parameters and then uploads them to the EHR through the help of a POC component and a server once the monitor is connected to the network. The POC component is required for episodic devices because the devices are moved from patient to patient. Without the mobile POC component, which links the patient to the device, there would be many patient association/disassociation issues, which could lead to incorrect data entered into a patient's electronic record. For example, if a patient is not disassociated from the vital-signs monitor, there is the potential that the next patient's data could mistakenly be entered into the previous patient's EHR.
Continuous stand-alone. Continuous stand-alone devices, such as ventilators, are used to monitor or treat a single patient over an extended period of time, but are typically not networked to any vendor-supplied central server (also referred to as a gateway). These devices are similar to episodic devices and require a POC component that is stationed either in the patient's room or attached to the medical device. For example, a ventilator that transports with the patient would include a POC component that is affixed to the ventilator (mobile), whereas a ventilator that remains within a patient room would have a POC component fixed on or near the medical device. Mobile POC components transmit data wirelessly, whereas fixed POC components typically transmit data via a wired local area network connection.
Continuous-networked devices. Continuous-networked devices, such as physiologic monitors and networked infusion pumps, monitor or treat a single patient over an extended period. Typically, these are connected to a vendor-supplied central server or gateway, which ultimately means no POC component is necessary. Data can be translated directly through these servers and gateways over the hospital network. Since there is no POC component, patient association/disassociation relies on entering patient identification into the medical device.
Because the majority of medical devices cannot transfer data directly to the EHR, medical device connectivity solutions have become a hot topic throughout health care. Health care facilities are hoping that these solutions will facilitate meaningful use requirements by ensuring accurate documentation and timely data transfer.
Structural consideration
Finally, what does this mean for the health care facility? Ultimately, how solutions will affect workflow and a facility's physical structure will have to be considered. Rooms need to be carefully planned, with special consideration to placement of equipment. Patient care rooms will require more wall outlets and network jacks to ensure that medical devices are always connected to the network and that data are being transferred. Cable management throughout the facility also will be important.
Unless the facility has a superb wireless infrastructure, certain medical devices will have to be hardwired into the network. And even if the facility does have an exceptional wireless infrastructure, there must be action plans for periods when these systems are down. Imagine the backlash from a physician who misdiagnosed a patient because test results were not transferred immediately due to a wireless issue.
A facility's infrastructure also will be impacted as more information flows over a hospital's network. It is likely that additional conduits and a complete infrastructure upgrade will be needed. Even if a facility already has decided on a software-based connectivity solution, it is recommended that all current and future needs be discussed ahead of time.
Complicated process
Integrating medical devices into an EHR is more complicated than plug and play.
Important considerations include:
- how the new solution will affect workflow;
- how it will affect the facility's physical requirements;
- how it can adapt to future infrastructure changes.
To ensure that all bases are covered, health facility biomedical engineers, clinicians and information technology personnel all should be involved in purchasing decisions.
Scott DiDonato is an associate consultant in the applied solutions group at ECRI Institute, Plymouth Meeting, Pa. He can be reached at sdidonato@ecri.org.
| Sidebar - What is an MDDS? |
| A medical device data system (MDDS) has the ability to transfer, store or display medical device data. It also can convert data into specific formats within preset specifications. However, MDDS devices cannot control or alter functions of any medical devices. Essentially, an MDDS is a product that can transfer, store or display data without modifying it or changing how it is displayed. It cannot include alarms intended to notify clinicians of a patient's condition. However, transferring alarms from a medical device to an electronic health record (EHR) is acceptable along with alarms that are intended to alert employees of MDDS problems. An MDDS is considered a Class I medical device, which the Food and Drug Administration (FDA) classifies as having the lowest risk for patients and does not require clearance before marketing. Certain medical device connectivity solutions fall within the Class I distinction, specifically ones that strictly store and transfer medical device data to the EHR. The connectivity solutions that do not fall under Class I distinction are considered Class II devices. This is typical for devices that were on the market before the MDDS ruling was finalized. Health care facilities that attempt to create their own connectivity solutions should be aware of their requirement to register their organization with the FDA. Not only must they register their organization, but they also must list their MDDS products, comply with FDA regulations and report any MDDS-related problems. It is recommended that a health care facility verify the regulatory classification of any connectivity solution or service under consideration to avoid any repercussions. |




